Fermentation is the central biochemical step in whisky production, in which yeasts in the mash convert sugar into alcohol and CO₂. In this section, you will learn how duration, temperature and yeast strain influence the development of flavours such as esters and higher alcohols and why precise control of the fermentation process is crucial for the subsequent flavour profile. We look at typical fermentation processes, explain the differences between short and long fermentation times and how to create optimal conditions to release the full potential.
The fermentation process
When the fermentation process is initiated, yeast is added to the wort to start the conversion of sugar into alcohol, releasing carbon dioxide (CO₂).
We can smell, see and feel this reaction!
The resulting alcohol odour is clearly recognisable, but you should avoid getting too close to the fermentation vessel due to the rising CO₂. As it rises, it causes clearly visible foam on the surface of the mash. The carbon dioxide evaporates into the air. Some distilleries that have a closed fume cupboard capture these gases. The heat generated during this process can be felt when you place your hand on the fermentation vat. It is the duration and temperature of the fermentation process that creates the complex flavours of the whisky, especially in the second part of the fermentation when the activity of the yeast decreases. Primary fermentation ends when the yeast can no longer convert sugar and the alcohol concentration in the wort becomes too high. At this stage, when the alcohol prevents the yeast from becoming active, various bacteria start to work.
Lactic acid bacteria
They were already "inhabitants" of the malt before mashing and therefore, unlike yeast, do not need to be added separately. The chemical reactions triggered by these bacteria lead to new compounds. These are acids, aldehydes, esters and long-chain alcohols. Although they only make up a small proportion, they influence the flavour and body of the wort and are not lost during subsequent distillation!
The duration of fermentation varies from distillery to distillery. It is completed after 48 to 96 hours and the mash has an alcohol content of around 6 to 10 per cent. This is enough for a good beer, but not for a whisky.
Anyone interested can find the biochemical background below.
The yeast
It's the yeast that does it! It feeds on sugar and produces not only alcohol, but also valuable aromas and flavours. During fermentation, heat and bubbles are created, which produce the characteristic 'bubbling'.
Mashing the malt produces a sugar solution, the so-called wort. A fermentation process is required to convert this sugar into alcohol, which is why yeast must be added. As yeast does not tolerate high temperatures, the wort is cooled to around 20 °C before the yeast is added to the fermentation vat. The yeast can either be added directly to the wort or dissolved and prepared in a small additional stirrer to initiate faster fermentation
There are different types of yeast used by brewers and distillers, but they all belong to a species called "Saccharomyces cerevisiae". Yeasts are fungi, not bacteria! Brewer's yeast develops more aromatic flavours, distiller's yeast can produce more alcohol. Most whisky distillers use a mixture of brewer's and distiller's yeast because experience has shown that it produces the best results in several respects. For example, a particular yeast strain ensures fast and complete fermentation, produces positive flavours and minimises foaming.
The amount of yeast accounts for 2 to 2.5 percent in the fermentation vat, but the distillery yeast can convert one tonne of malt into 400 litres of alcohol.
The fermentation vat
The fermentation of beer, wine and whisky generally takes place in a fermentation vat. It can be made from different materials. High resistance to alcohol and fruit acids is important.
Traditional wooden fermentation vats
Fermentation vats are traditionally made from wood. They are often made from Oregon pine, which grows very tall, has few knots and a very fine wood structure. However, there are also fermentation vats made from larch or cypress, which are covered with lids to prevent bacteria from entering and the mash from overflowing if it rises too high during fermentation. Rotating blades are fitted in the fermentation vats as an additional means of preventing foaming.
Wood is an organic material and an ideal host for bacteria. For this reason, wooden fermentation vats must be cleaned particularly carefully and thoroughly to avoid undesirable side effects. Cleaning is carried out both by strong steaming and by using chemical solutions. This is not only necessary for traditional wooden fermentation vats, but also for those made of iron or steel. However, the risk of persistent bacterial "guests" is particularly high with wooden vats.
So why still use wooden vats? It is claimed that fermentation in wooden vats provides flavours due to interactions. However, when you consider how often the vats are used, how small the surface area of the wood that comes into contact with the mash is and, finally, how short the fermentation time is, you can imagine how small this influence can ultimately be.
But there is one argument in favour of wooden fermentation vats that trumps all other arguments, however logical and competent they may be: wooden vats simply look better in our eyes and fit in with our ideas of traditional whisky making!
Old cast iron fermentation vats
In the 19th and 20th centuries, some distilleries were looking for alternatives. They wanted to use materials that were easier to clean and had a longer lifespan. Cast iron was the answer at the time, and these heavy fermentation vats can still be seen in some distilleries.
New stainless steel fermentation vats
Stainless steel was the great invention at the beginning of the 20th century that soon revolutionised most branches of industry. You will see stainless steel in every distillery you visit, even if they still use traditional methods. Some use it only as a tank for the raw spirit, others have recognised its considerable advantages and have replaced many other containers and apparatus, such as fermentation vats, with stainless steel vats.
Stainless steel vats are the optimal alternative to wooden fermentation vats when it comes to applying cleaning solutions and keeping bacteria and fungi out through a closed tank. Modern stainless steel vats have automatic cleaning systems so that fewer chemical cleaning processes are required.
Primary fermentation ends when the yeast can no longer convert sugar and the alcohol concentration of the wort becomes too high. At this stage, when the alcohol prevents the yeast from becoming active, various bacteria start to work, especially lactic acid bacteria. They were "inhabitants" of the malt before it was mashed, so unlike the yeast, they do not need to be added separately. The chemical reactions triggered by these bacteria lead to new compounds. These are acids, aldehydes, esters and long-chain alcohols. Although they only make up a small proportion, they influence the flavour and body of the wort. As most of them are not lost during the subsequent distillation process, it would be a big mistake not to pay attention to them.
Biochemical background
Whisky is by definition a drink distilled from grain. But how is grain turned into alcohol? Through fermentation, of course. Before this happens, a long chain of chemical and biological reactions has to take place.
What happens inside the fermentation vats when yeast is added to the wort? Let's call it chemistry! It's a very simple reaction, but the result is fascinating: C6H12O6 -> 2 x C2H5OH + 2 CO2 + heat.
This formula in words: There are glucose molecules in our wort. The yeast now spits them out and each molecule provides two ethanol molecules (that's the alcohol we need), two carbon dioxide molecules and heat.
A detailed explanation follows.
Sugar
The grain consists primarily of starch. The other components are proteins, fats and trace elements. Starch is the starting material for alcohol production. To make it easier to explain the chemical relationships, however, I would like to start with sugar rather than starch. Sugar and starch both belong to the group of carbohydrates. Once you have understood the basic properties of sugar, it is easy to draw conclusions about starch.
Historically, our local ancestors only knew bee honey and fruit as sources of sugar. This changed abruptly with the discovery of the world, when cane sugar came to us from the southern latitudes. It was not so long ago that sugar beet was introduced to us as a source of sugar. From the middle of the last century, more and more beet sugar factories were built in our latitudes. Today we produce sugar in beet and starch sugar factories and also import considerable quantities of cane sugar into the European Community (suppliers e.g. South Africa and Mauritius).
Everyone knows some forms of sugar from food advertising. In addition to the sugars listed below, there are many other sugars whose chemical structure is similar.
| Designation | Chemical name | Formula |
|---|---|---|
| Cane sugar | Sucrose | C12H22O11 (=2*C6H12O6-H2O) |
| Dextrose | glucose | C6H12O6 |
| Fructose | fructose | C6H12O6 |
| Milk sugar | Lactose | C12H22O11 |
| Malt sugar | maltose | C12H22O11 |
Fructose
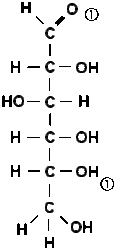
The sugars that are important to us, glucose and fructose, have six carbon atoms (C6H12O6). However, there are also sugars with five or seven carbon atoms (example: ribose) in which an H-C-OH unit is missing or has been added to the chain.
A fruit sugar (fructose) has a slightly different structure in the chain. The second H-C-OH group is replaced by a ketone group (C=O). The aldehyde function at the end of the chain is missing.
What makes sugar sweet?
It is the OH groups that react with the receptors on our tongue. However, it is not the quantity of OH groups that is decisive, but rather the position of these OH groups in space, which can only make contact with our receptors on the tongue in a certain position. There are substances whose OH groups are arranged similarly in space (sweeteners, glycol, etc.) and therefore also taste sweet.
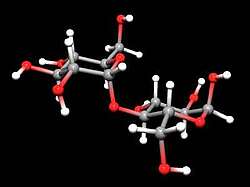
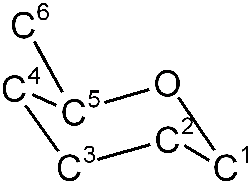
The C1 and C5 atoms of sugar can combine (add) via the double-bonded oxygen atom of the upper aldehyde group to form a ring of five carbon atoms and one oxygen atom. No atom is split off, but all atoms are incorporated into the new ring structure. The sixth carbon atom sticks out to the side of the ring. However, the ring is not flat in space, but rather bent in the shape of a chair. This ring structure is energetically more favourable than the free chain. Statistically, a mixture of 99% rings and 1% chains is found in a glucose solution.
Strength
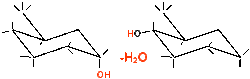
Glucose is now converted into starch by linking several of these rings to form chains by splitting off a water molecule. The water is split off between the C1 and C4 atoms. This compound is the malt sugar (see picture on the right).
This formula describes the chemically pure starch called amylose. Pure amylose is spatially coiled. In nature, starch does not have such a regular structure. There are not only chains but also branches.
If beta-glucose is linked using the same method, the result is cellulose, as found in the wood of whisky barrels. The cellulose molecules are long chains that can lie next to each other and bind via hydrogen bonds. This is why cellulose is fibrous and more stable than starch flour.
The enzyme amylase from barley can now break down the starch of the cereal grain at the O-bonds. However, it cannot do anything with cellulose. It cannot recognise the beta-forms. Goats, on the other hand, are able to break down cellulose into sugar with the help of microorganisms in their intestines.
The enzyme attacks the chains, splits them and separates two sugars (dimers and maltose) from the ends of the strand. Once the entire chain has been split, only chains of 2 and 3 (trimers) remain. The enzyme cannot break down the trimers any further.
SPECIAL FEATURE: The enzyme amylase is found exclusively in barley malt. However, it can break down not only barley starch but also starches from other types of grain into sugar. For this reason, the mash for bourbon and grain whisky usually contains 10% barley malt.
Large grain distilleries, starch sugar factories and American whiskey distilleries utilise a special feature of starch. Starch can be split acid-hydrolytically with the addition of heat. In the boilers of the bourbon distilleries, the corn is boiled in an acidic environment (sour mash) at a slight overpressure at 105 °C for 25 minutes (1.14 bar = 2 psi overpressure), thus accelerating the breakdown of the starch. This can replace the use of barley.
Alcoholic fermentation
The last step from grain to alcohol is alcoholic fermentation. What is alcoholic fermentation? Let's just play dumb ... (loosely based on Heinz Rühmann - 'Die Feuerzangenbowle')
Alcoholic fermentation is not carried out by enzymes, but rather by yeasts. Yeasts are not bacteria but fungi. Yeasts can be found everywhere in nature. Especially in autumn, when the fruit ripens, their spores fly through the air in large numbers. They carry out a simple chemical reaction in the sugar/water solution according to the following formula:
Yeast
The yeast splits a glycose molecule and produces two ethanol molecules(alcohol) and two carbon dioxide molecules and energy in the form of heat per ring. In addition, fruity flavourings (esters) are produced, which give the whisky its great variety of flavours. The yeast fungus cannot harm the pure starch. It remains unaffected by the fungi.
In Scotland, two different dry yeasts (baker's and brewer's yeast) are generally used. The first of the two ensures that fermentation starts quickly. At the same time, the wash is acidified. The second yeast works better in an acidic environment and only reaches its maximum performance later. It ensures that a high alcohol content is produced in the wash.
In the USA, the fruity esters of special yeasts are particularly important in whiskey production. Every bourbon therefore has its own yeast(s). They were isolated from wild yeasts and patented by the companies. All the yeasts used are produced in large quantities in our own propagation facilities and are also added in liquid form.
Carbon dioxide
The carbon dioxide rises in the fermenting, bubbling solution and disperses into the air. The ethanol produced accumulates in the solution. The yeast lives off the energy released. This process continues until either all the sugar has been consumed (typical for whisky) or until the alcohol concentration has risen to such an extent that the yeast fungus kills itself with its own products (typical for wine).
Vinegar - Acetic acid - Vinegar bacteria
Every distiller, brewer or winemaker has to deal with vinegar bacteria as a matter of necessity. Just like yeast fungi, vinegar bacteria are found in the wild. They feed on alcohol and produce acetic acid. Once a fermentation container (wash back, fermenter) is infested with vinegar bacteria, the entire filling can no longer be used. For this reason, the wash backs in Scotland are cleaned with chemical substances and in the USA the fermenters are even sterilised at high temperatures. The basic chemical reaction carried out by the vinegar bacteria looks like this:
As the reaction requires oxygen, an infection with vinegar bacteria can usually be avoided under strict exclusion of air. In addition to the vinegar bacteria described here, there are special bacteria that first produce alcohol themselves before they produce acetic acid. However, there are also more highly developed fungi that compete with these vinegar bacteria and can also react from starch to acetic acid. Nature still has many wonders in store for us. Until they are discovered, we should simply enjoy the products of nature.
Conclusion
Fermentation is at the heart of every whisky production process: through targeted yeast selection and controlled fermentation time, malt sugars are transformed into aroma and alcohol carriers that give the subsequent distillate its characteristic variety of flavours. A deeper understanding of this step helps beginners and connoisseurs alike to experience the nuances of their favourite dram even more consciously.











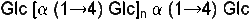
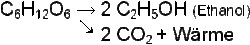

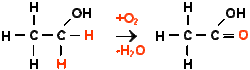

















To comment, you must be logged in